Zebrafish (center) are used to study ReNU syndrome, for example examining brain changes (left) and behavior differences (right), providing insights into the disease.
It’s time to advance research towards diagnosis & treatment options!
Participate in ReNU patient registries and research opportunities.
IMPORTANT: If enrolling a ReNU patient in research, from ANY country, please Create a Clinical Research ID (CRID)! This is crucial to using the research data collected across platforms and research programs. Example for CRID registration: Disease Name: ReNU Syndrome | Gene: RNU4-2 | Variant: n.64_65insT (or your variant)
Once you create a CRID, it’s yours to hold on to! We encourage you to share it with each clinical research study that you enroll your ReNU child in.
If there was a treatment for your family member with ReNU syndrome, what would you want it to address? Your voice and perspective matters! This poll helps guide us towards identifying clinical endpoints for future potential treatments for ReNU.
Research Opportunities
-

Rare-X Registry
International: YES
Rare-X is a critical registry and ALL are welcome to participate. Developing treatment options requires that we have robust data that is continually updated.
-

Citizen Health
Through our partnership, securely organize medical records into a private, digital profile—no site visits required—get instant answers based on YOUR personal journey, and help power research that could lead to new treatments.
Your real-world experiences could contribute to critical natural history studies, enabling researchers to better understand ReNU, uncover patterns, and accelerate progress toward breakthroughs.
-

Clinical Health Survey
ENROLL- email NGHI@northwell.edu
1. Email research team (available Monday-Friday, 9am-5pm ET to answer questions).
2. Receive electronic consent form; sign & return, receive survey link
3. Survey takes ~45 minutes to complete. Questions focus on pregnancy history, genetic testing, medical history (including a brief review of each body system), and family history.
OVERVIEW:
Survey is led by Dr. Ian Krantz, Kelsey Crocker, and Asbaa Khan with the support and expertise of ReNU Syndrome United board members and advisors.
Goal: Perform a cross-sectional study by collecting and analyzing information on the spectrum of features that individuals with ReNU syndrome experience and how these features change or progress over time. Understanding the spectrum of symptoms and their progression empowers parents and guardians to provide optimal care.
Purpose: This study will contribute valuable data to researchers investigating and developing treatments for this condition. Furthermore, it will enhance clinicians' and genetic counselors' understanding of this novel diagnosis, improving recognition of the characteristic features of ReNU syndrome.
-

INDEED Study
ENROLL - email zafiirah.baurhoo@mssm.edu
If you suspect a family member might have ReNU, you may be eligible for FREE testing. If your family member already has a clinically confirmed genetic diagnosis of ReNU / RNU4-2 or RNU2-2 related disorder and you are interested in participating in the INDEED study, please request a clinical appointment with Dr. Barbosa via MSSMClinicalGenetics@mssm.edu.
Drs. Ernest Turro and Mafalda Barbosa at the Icahn School of Medicine at Mount Sinai have established the INDEED research study to investigate ReNU and other newly discovered genetic conditions. Dr. Turro leads one of the research teams that discovered that mutations in RNU4-2 cause ReNU (Greene et al. 2024). His group also discovered that mutations in a closely related gene, RNU2-2, cause a similar neurodevelopmental disorder (Greene et al. 2025). Dr. Barbosa has over 15 years of experience as a clinical geneticist with a focus on diagnosis and medical management of patients with rare neurodevelopmental disorders. The INDEED study offers free research-use DNA sequencing of the RNU4-2 and RNU2-2 genes.
-
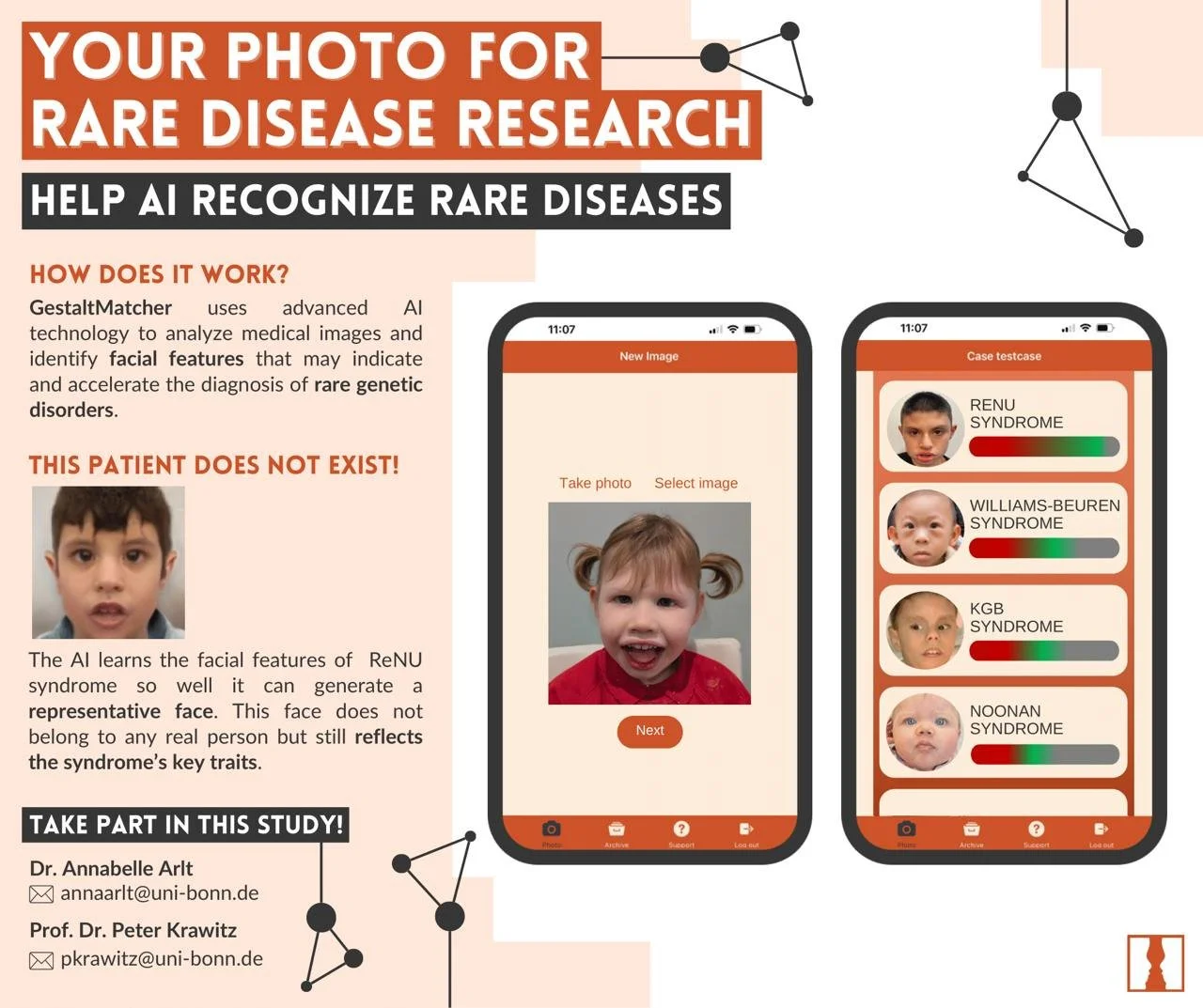
GestaltMatcher
ENROLL - Email annaarlt@uni-bonn.de
International: YES
Contact Dr. Annabelle Arlt or Dr. Peter Krawitz (pkrawitz@uni-bonn.de )
GestaltMatcher uses advanced 2D Al technology to analyze medical images and identify facial features that may indicate and accelerate the diagnosis of rare genetic disorders.
-
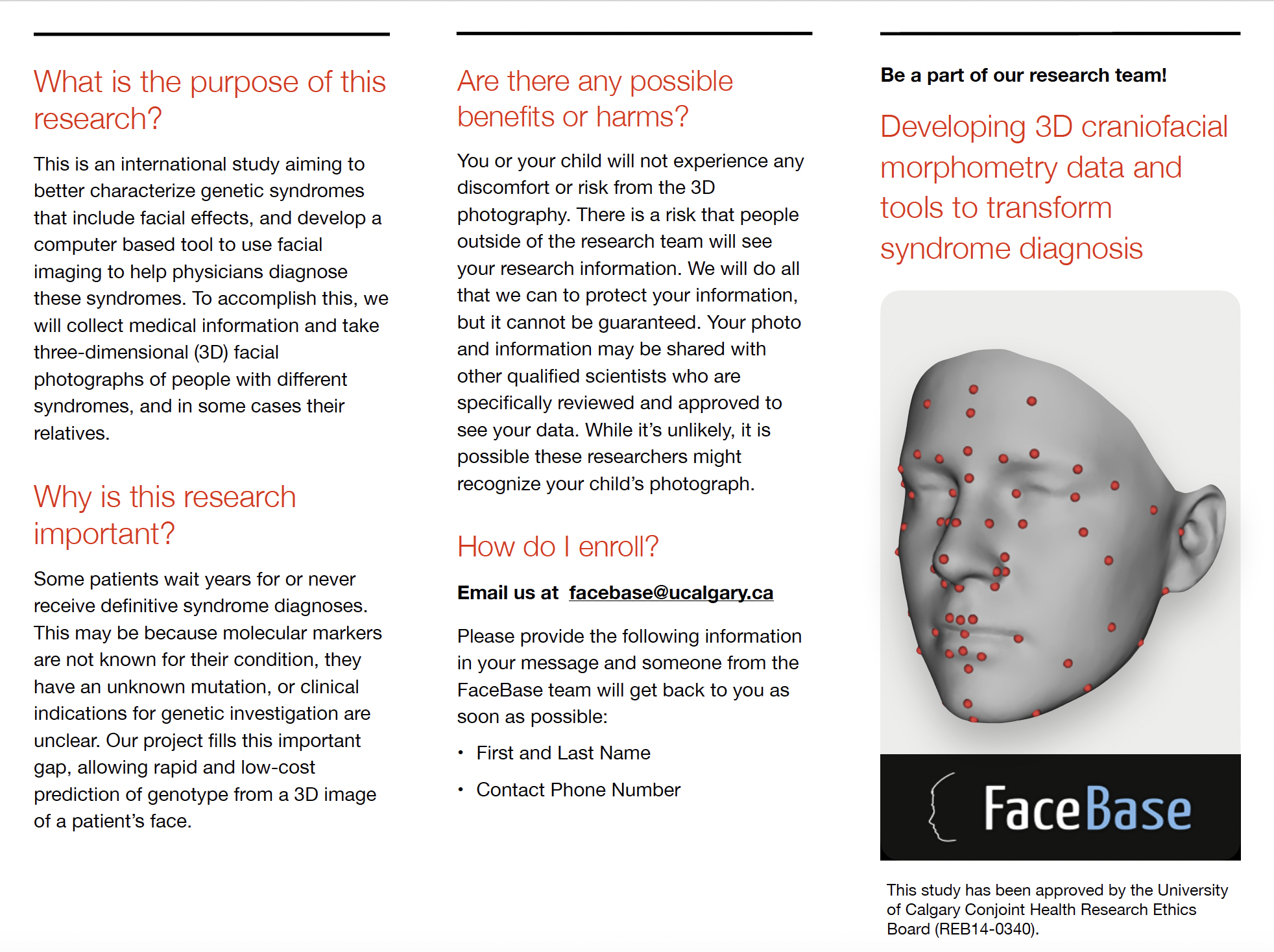
FaceBase
ENROLL: email facebase@ucalgary.ca
International: YES
3D International Facial Recognition Study. Participation requires <20 minutes. More information at https://hallgrimssonlab.ca/.
The FaceBase team from the University of Calgary is conducting an international study aiming to better characterize genetic syndromes that include facial effects. This includes a computer-based tool using facial imaging to assist physicians in diagnosing syndromes and assessing severity to identify the likelihood of disease-related complications.
Many patients wait years for a definitive syndrome diagnosis or never receive one. Even with a diagnosis, understanding severity and the likelihood of disease-related complications is crucial for clinical decision-making. This may arise due to the absence of known molecular markers for their condition, the presence of an unidentified mutation, or unclear clinical indications for genetic investigation. The FaceBase project aims to bridge this critical gap by enabling the rapid and cost-effective prediction of genotype and associated condition severity through a 3D image of a patient's face. During study participation, video recording is used to minimize challenges that can arise from movement and sensitivities during flash photography. This video is used to select the best moment/image to generate a 3D picture of the participant.
-

STARS Epilepsy Study
International: YES
Criteria: experience prolonged epileptic seizures (>3 minutes) to join this clinical research study. Testing an inhaler containing an investigational drug designed to potentially stop a prolonged seizure once it has begun.
The following languages are supported: US English and US Spanish, Czech, Bulgarian, AUS English, Italian, Hungarian, French, German, Polish, Spain Spanish, UK English
FAQs
What is a Registry?
Registries are organized systems to collect and store standardized data about RNU4-2 / ReNU Syndrome individuals, enabling researchers to study treatment options, disease progression, and other factors related to healthcare over time.
Why do I need a CRID - Clinical Research ID?
This is crucial to using the research data collected across platforms and research programs. When you share it with a research study, it will be used to anonymize your ReNU child, so that researchers may learn from the data you share: they associate your child with a clinical research ID instead of getting names or personally identifiable information about them. If you enroll in multiple research studies, the hope is that by cross-referencing your CRID, you may not need to repeatedly answer the same questions you’ve already answered in previous studies. As an organization, we are encouraging lots of research into ReNU so that it can fuel the development of potential treatment options.
How can I opt in to be contacted for research opportunities?
When you register here on our RNU4-2 map, there is a check-box to opt in to be contacted for future research opportunities.
ReNU Syndrome United is proud to serve as a conduit to connect families with research opportunities. Participation in any study is voluntary, and individuals should carefully review all consent forms and study details before enrolling. All participation is at the individual/family’s own risk.
To get meaningful data about the progression of RNU4-2 / ReNU syndrome, we need you to join Citizen Health!
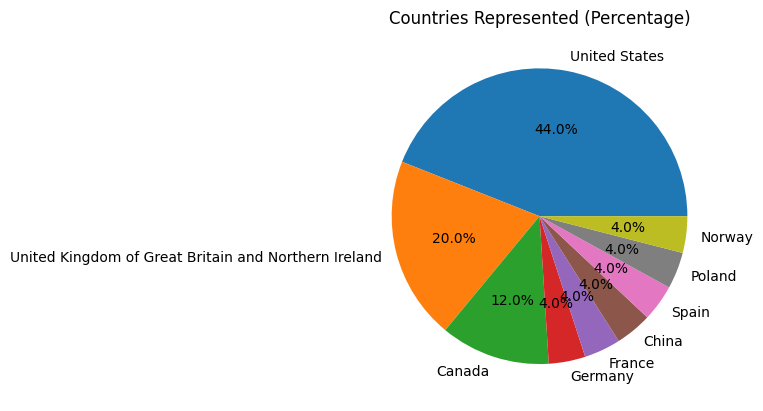
Snapshot of registrants as of January 26, 2026:
What’s important to the RNU4-2 Community? Take the Treatment Priority Poll
Analysis of results as of January 26, 2026:
Top 10 priorities in rank order, based on the weighted caregiver poll results:
Absent / delayed speech & communication
Epilepsy / seizures
Brain abnormalities
Mobility
Feeding
Low muscle tone (hypotonia)
Bone fragility
Drooling
Lower gastrointestinal issues (e.g., constipation)
Sleep disturbances